The MERCURI-2 trial
A multicentre study funded by The Netherlands Organisation for Health Research and Development (ZonMw) through Programme “Goed gebruik geneesmiddelen” Project number: 10140022010003.
This page provide information on the progress and results of our study for patients, participants, members of the public as well as interested colleagues.

Information for participants
Thank you for your participation in the MERCURI-2 trial! If you wrote down your email address on the informed consent form, we will inform you about the results after the trial is finished.
Bedankt voor uw deelname aan de MERCURI-2 studies! Als u uw email adres heeft opgegeven op het patienteninformatie en toestemmingsformulier, houden we u op de hoogte van de studie resultaten nadat de studie voltooit is.
Progression
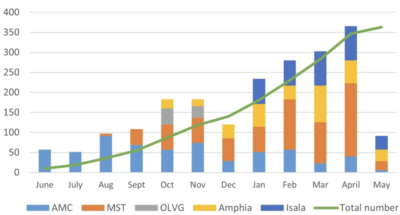
At last count, 363 participants were enrolled in the trial that is ±45% of the 784 required participants. Patients are currently at 5 Dutch cardiothoracic surgery centres, with an overall inclusion rate exceeding projections and planning.

Dr. Bas Gerritse
Cardiac anesthesiologist
Dr. Thierry Scohy
Cardiac anesthesiologist
Michelle Verhagen
Research coordinator
Maaike Thio
PhD student

Dr. Ferdinand Snellen
Anesthesiologist-intensivist
Dr. Martijn Tolsma
Cardiac anesthesiologist-intensivist
Henrico Wesselink
Research supervisor anesthesiology and IC
Alice Pap-Brugmans
Research nurse
Yvonne Jordens
Research nurse

Dr. Jeroen Wink
Cardiac anesthesiologist
Jesper Hjortnaes
Cardiothoracic surgeon
Eline Bruggemans
Clinical researcher

Dr. Marc Godfried
Anesthesiologist
Rients de Boer
Cardiac anesthesiologist-intensivist

Dr. Magiel Voogd
Cardiac anesthesiologist

Nelson Oliveira
Cardiac anesthesiologist-intensivist
Ed Niesten
Cardiac anesthesiologist-intensivist
Study Overview
Background
Acute kidney injury (AKI) is one of the most common major complications after cardiac surgery and is associated with postoperative morbidity and mortality. Currently, no effective therapy exists to reduce the incidence of postoperative AKI. Sodium-glucose transport protein 2 (SGLT2) inhibitors may reduce the incidence of AKI. Therefore, we hypothesize that perioperative SGLT2 inhibition will reduce the incidence of acute kidney injury.
Study design
We designed a multicenter randomized, placebo-controlled, triple-blinded, superiority trial. We aim to include 784 patients undergoing cardiac surgery between the age of 18 to 90 years and stratify for sex and type 2 diabetes in a 1:1 ratio. Patients will receive either dapagliflozin 10mg or placebo from the day before surgery until two days thereafter. The primary outcome is the incidence of acute kidney injury according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria.

Funding
This trial is funded by The Dutch Organisation for Health Research and Development (ZonMw) through the programme “Goed gebruik geneesmiddelen”. Project number: 10140022010003.


Collaboration
During the trial preparation, we collaborated closely with the Dutch patient associations for kidney disease, the “Nierstichting” and cardiovascular disease, “Harteraad”, to develop the trial’s design and patient information materials.